What Statement Best Describes Metallic Bonding
The attraction between nuclei and electrons B. Which statement best describes the basis of the band theory of metallic bonding.

Question Video Identifying The Structure Of A Metallic Bond Nagwa
- 8111946 ashirayen ashirayen 05122020 Science.

. Chemistry questions and answers. Check all the boxes that describe the electron sea model. Metallic bonding may be described as the sharing of free electrons among a lattice of positively charged metal ions.
That is to say instead of orbiting their respective. And also there is no single. Which statement best describes the basis of the band theory of metallic bonding.
--It is the simplest metal bonding model. Which of the following statements best describes metallic bonding. Which statement best describes metallic bonding.
--Metallic bonding results from the transfer. Molecular orbitals overlap to form atomic orbitals in which the. The attraction between positive ions and electrons C.
Which statement best describes the basis of the band theory of metallic bonding. A metallic bond is not actually a bond but it is an interaction between conduction electrons and metallic nuclie. Which of the following statements best describes metallic bonding.
Advertisement eycor8844 eycor8844 Answer. A Metal transfers or donates its electron to a non-metal forming an ionic compound B. Electrostatic attractions between a lattice of positive ions and.
Atomic orbitals overlap to form molecular orbitals in which the valence electrons of the atoms travel. A Metal transfers or donates its electron to a non-metal forming an ionic compound B. O Anions are held together by electrons O.
--It is the most complicated metal bonding model. A metallic bond is a bond between. Electrons flow easily between metal nuclei.
Which statement best describes the energy change as bonds are formed and. Electrostatic attractions between oppositely charged ions B. 1 point Which of the following statements BEST describes the formation of a metallic bond.
Explain how metallic bonding between copper atoms can account for each of these properties. Which statement best describes the attraction present in metallic bonding. A metallic bond is a type of chemical bond formed between positively charged atoms in which the free electrons are shared among a lattice of cations.
Which of the following statements best describes metallic bonding. Molecular orbitals overlap to form atomic orbitals in which the valence electrons of the atoms travel.

Bonding In Metals The Electron Sea Model Introduction To Chemistry

Metallic Bonds Properties Examples Explanation Of Metallic Bonds
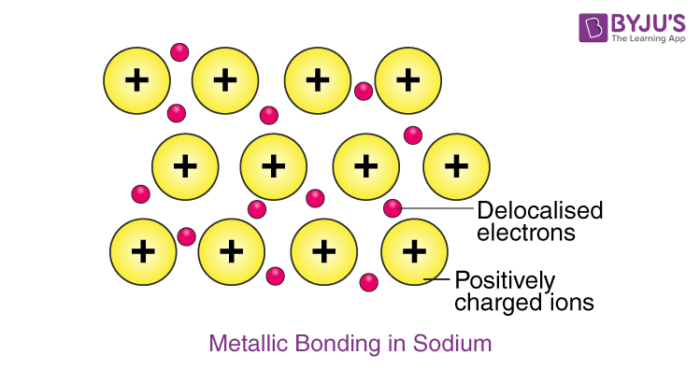
Metallic Bonds Properties Examples Explanation Of Metallic Bonds

No comments for "What Statement Best Describes Metallic Bonding"
Post a Comment